Why, when and how to start treating PPF* with OFEV®
OFEV is indicated in adults for the treatment of idiopathic pulmonary fibrosis (IPF). OFEV is also indicated in adults for the treatment of other chronic fibrosing interstitial lung diseases (ILDs) with a progressive phenotype. OFEV is indicated in adults for the treatment of systemic sclerosis associated interstitial lung disease (SSc-ILD).1
PPF* = Progressive Pulmonary Fibrosis. In this material PPF refers to progressive, fibrosing lung diseases excluding IPF. The term PPF is used when referring to INBUILD results and the ATS/ERS/ JRS/ALAT Clinical Practice guideline (2022) due to overlap in their criteria for progression and comparable clinical outcomes in PPF and PF-ILD.2

Why to treat PPF with OFEV
OFEV reduce lung function decline.1,3
OFEV slows down the annual rate of decline in FVC by 57% in PPF1,3
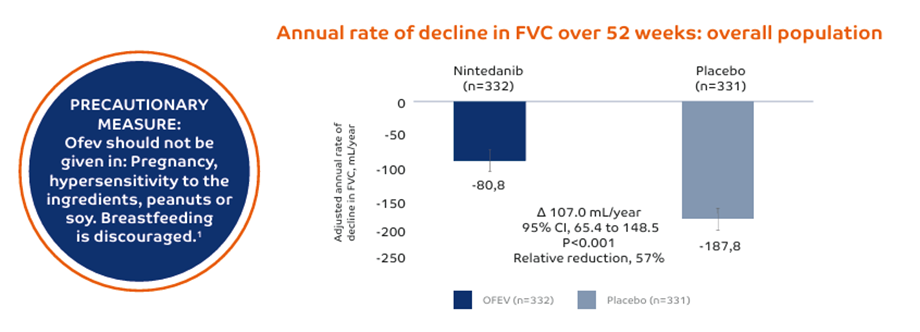
This figure is adapted from Flaherty KR, Wells AU, Cottin V et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N Engl J Med 2019, 381:1718-1727. Supplementary Material
Study design: 663 patients randomized 1:1 to receive either OFEV or placebo over 52 weeks. Randomization was stratified by HRCT pattern (63% UIP-like fibrotic pattern; 38% other fibrotic pattern) based on central review. Part A was conducted during 52 weeks, Part B was a variable treatment period beyond 52 weeks, which was still conducted in a double blind setting until all patients completed Part A.1,3 The primary end point in INBUILD was the annual rate of decline in FVC, assessed over the 52-week period. The main secondary endpoints were absolute change in K-BILD at week 52, time until first exacerbation or death over the 52-week period and time until death over the 52-week period.3 Inclusion criteria, INBUILD: Patients met specific criteria for progression of ILD despite management in the 24 months before screening. >10% extent on HRCT, FVC ≥45% predicted, DLCO ≥30%-<80% predicted.3
REDUCE the risk of acute exacerbations with OFEV1,3
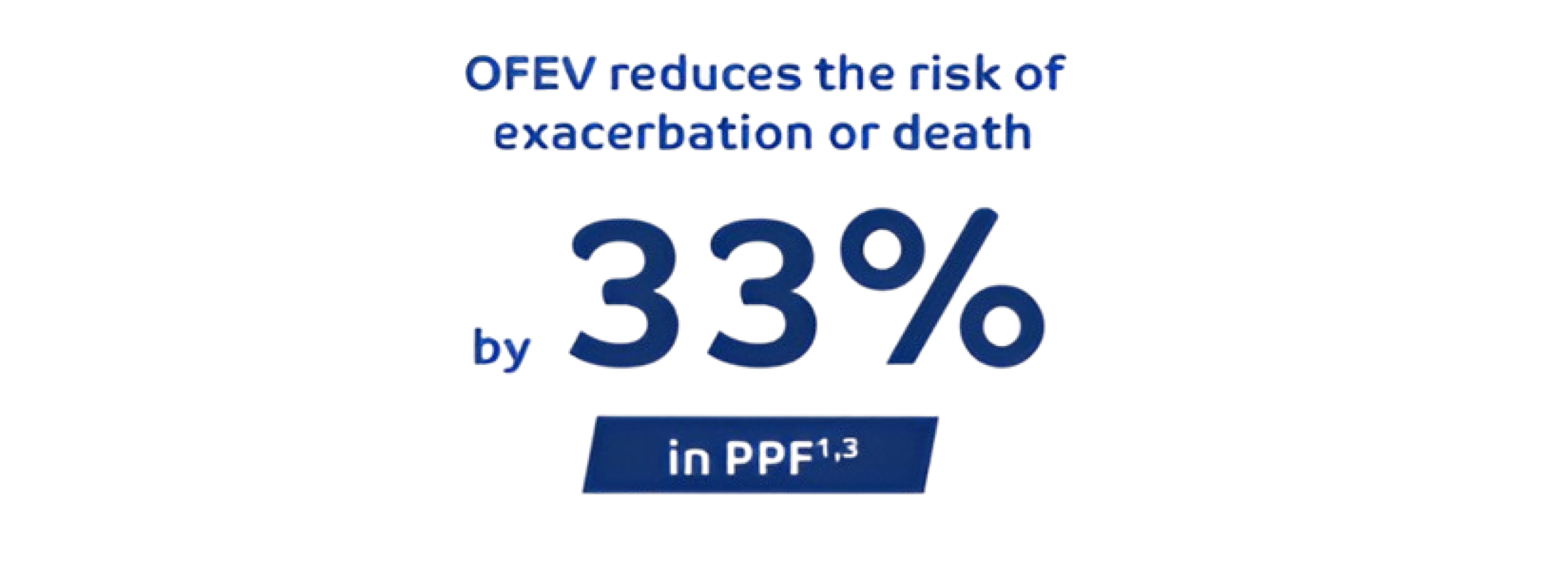
INBUILD: Time to first acute exacerbation of ILD or death in overall population over whole trial.1,3 Hazard ratio=0,67, 95% CI= 0.46, 0.98. Nominal P=0,04 Study design: 663 patients randomized 1:1 to receive either OFEV or placebo over 52 weeks. Randomization was stratified by HRCT pattern (63% UIP-like fibrotic pattern; 38% other fibrotic pattern) based on central review. Part A was conducted during 52 weeks, Part B was a variable treatment period beyond 52 weeks, which was still conducted in a double blind setting until all patients completed Part A.1,3 The primary end point in INBUILD was the annual rate of decline in FVC, assessed over the 52-week period.
The main secondary endpoints were absolute change in K-BILD at week 52, time until first exacerbation or death over the 52-week period and time until death over the 52-week period.3
PPF = Progressive Pulmonary Fibrosis.
When to treat PPF with OFEV
SUPPORT your PPF patient’s quality of life1,4
OFEV significantly reduced the worsening of cough* and dyspnea in patients with PPF1,4 Absolute change from baseline in dyspnea and cough symptoms scores at 52 weeks in the INBUILD trial1,3
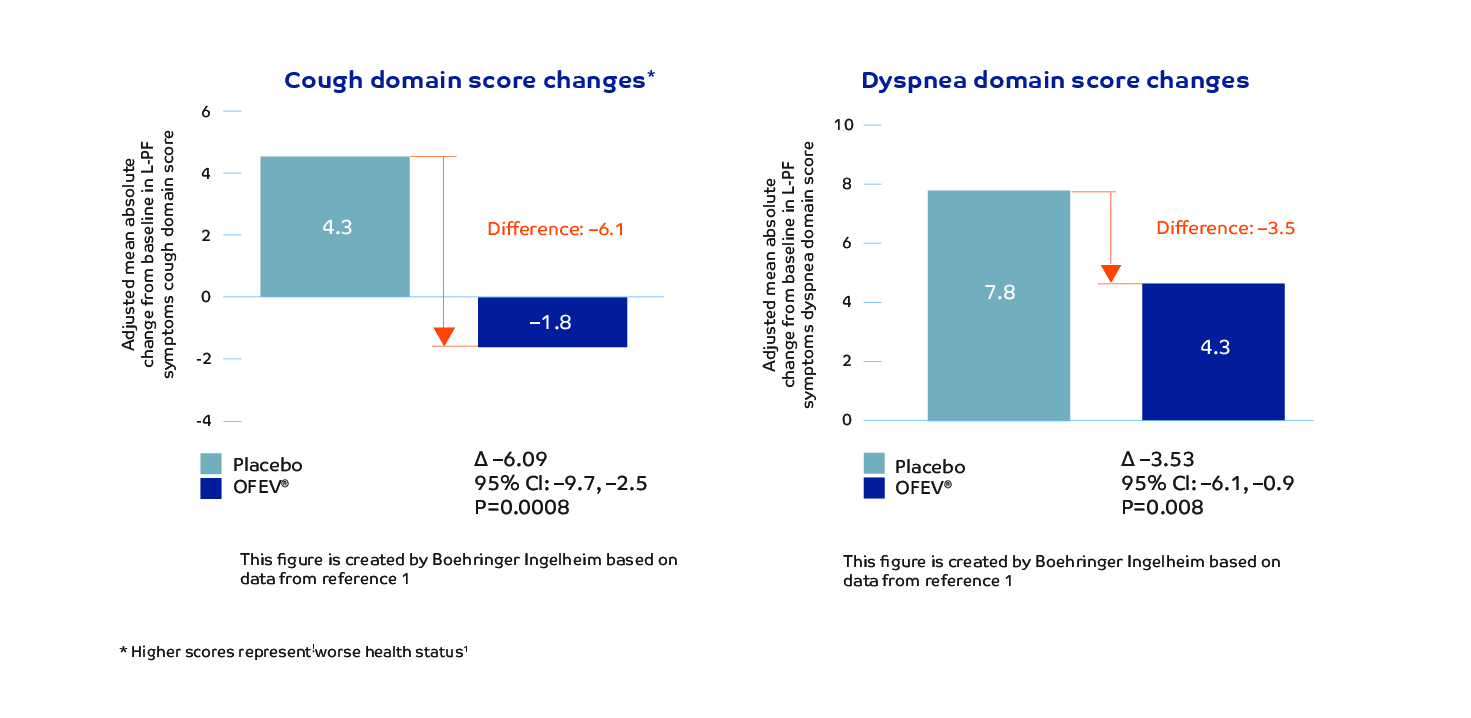
Results based on the Living with Pulmonary Fibrosis (L-PF) questionnaire assesses symptoms and quality of life in patients with fibrosing interstitial lung diseases (ILDs). Higher scores represent worse health status.5
*Cough domain scores meet the threshold for the smallest, clinically significant difference (MCID).4
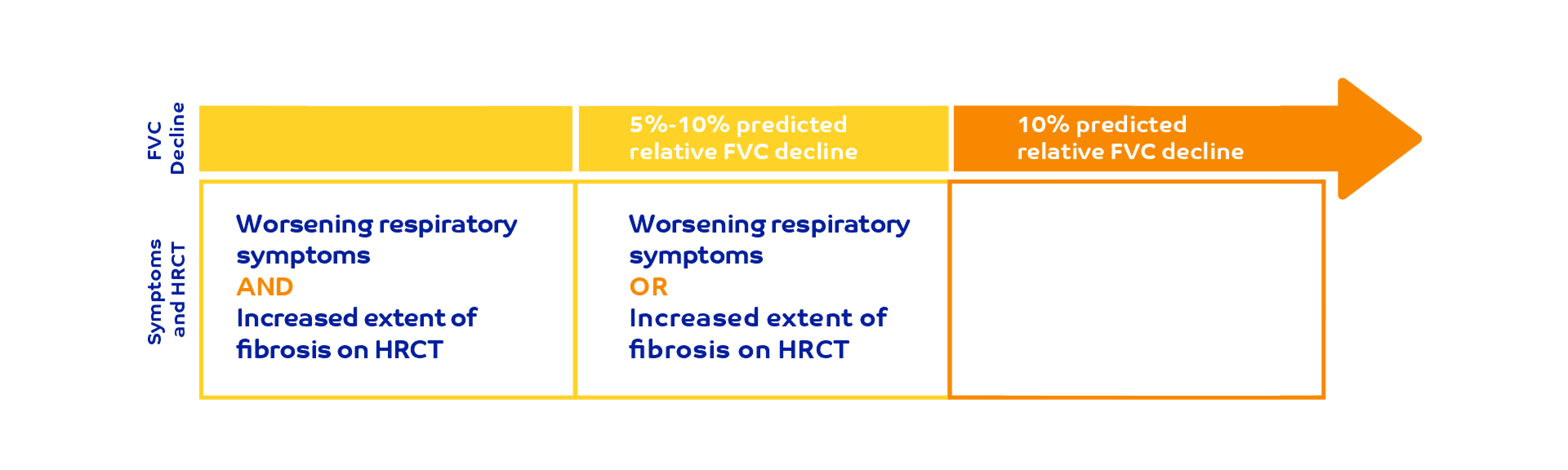
Criteria for ILD progression1,3
Patients were required to meet ≥1 of the following criteria for ILD progression in the 24 months before screening, despite management:
Consider treatment
Treat according to approved indication and defined progression criteria and guidelines1,3,8,11
Even if the patients use concomitant immunomodulatory therapy1,6,7
How to treat PPF with OFEV
Adults:
One 150 mg capsule twice daily with food.1

Packs and prices:
100 mg 60 pcs (blister) 15.903,90 NOK 150 mg 60 pcs (blister) 28.435,6
Refundable use:
H-prescription. Where national action programmes/national professional guidelines and/or recommendations from the RHS/LIS specialist group have been prepared, requisitioning must be made in accordance with these.
Conditions:
216 Reimbursement is only given by prescription from a hospital doctor or contract specialist.
Prescription group: C
See the Norwegian Pharmaceutical Compendium for the full summary of product characteristics.
Most common adverse events are:
Diarrhoea, nausea, vomiting, abdominal pain, decreased appetite, weight loss, and hepatic enzyme increase.1
OFEV side effects can be managed in most patients1
Dose modification with OFEV allows for the management of some adverse events while supporting continued clinical benefit.1,9
If your patient is reporting adverse reactions, consider:
Temporary treatment interruption or dose reduction to 100 mg twice daily.1
Once the event has resolved, reinstate to the optimal dose of 150 mg twice daily for higher clinical efficacy, as shown in the TOMORROW trial.1,9,10
If your patient is experiencing diarrhea, treat immediately with adequate hydration and initiate anti-diarrheal drugs, such as loperamide.1
Loperamide can be initiated at a dose of 2 mg once daily but if symptoms persist, the dose can be increased up to 4 mg four times daily.9
Obligatorisk informasjon:
Ofev® (nintedanib)
Indikasjoner: Voksne: Idiopatisk lungefibrose (IPF). Andre kroniske fibroserende interstitielle lungesykdommer (ILD) med en progressiv fenotype. Systemisk sklerose-assosiert interstitiell lungesykdom (SSc-ILD).
Dosering: 150 mg 2 ganger daglig med ca. 12 timers mellomrom. Bivirkninger håndteres ved symptomatisk behandling, dosereduksjon og midlertidig behandlingsavbrudd, inntil aktuell bivirkning tillater fortsatt behandling.
Kontraindikasjoner: Graviditet. Overfølsomhet for innholdsstoffene, peanøtter eller soya. Frarådes ved amming.
Forsiktighetsregler: Tett oppfølging av gastrointestinale symptomer, leververdier, nyrefunksjon og blødning. Forsiktighet utvises ved blødningsrisiko og forhøyet kardiovaskulær risiko. Bør ikke brukes ved alvorlig pulmonal hypertensjon. Dosereduksjon eller behandlingsavbrudd kan være nødvendig. Posterior reversibelt encefalopatisyndrom (PRES) er sett. Hvis PRES mistenkes skal nintedanibbehandling seponeres.
Interaksjoner: Samtidig bruk av potente P-gp-hemmere kan øke eksponeringen. Potente P-gp-induktorer kan redusere eksponeringen.
Utvalgte bivirkninger: Svært vanlige (>10%): IPF: Abdominalsmerter, diaré, kvalme, økte leverenzymer. PF-ILD: Abdominalsmerter, diaré, kvalme, oppkast, økt ALAT, økte leverenzymer, nedsatt appetitt. SSc-ILD: Abdominalsmerter, diaré, kvalme, oppkast, økte leverenzymer. Alvorlige bivirkninger sett ved ikke kjent, vanlig eller mindre vanlig frekvens uavhengig av indikasjoner:
Trombocytopeni, myokardinfarkt, blødning, aneurismer og artieriedissekjoner, kolitt, legemiddelindusert leverskade, nyresvikt, proteinuri, posterior reversibelt encefalopatisyndrom.
Pakninger og priser: Kapsler, myke (blister 60 stk): 150 mg kr 28 435,60. Kapsler, myke (blister 60 stk): 100 mg kr 15 903,90.
Refusjon: H-resept: Voksne: IPF og andre kroniske fibroserende ILD-er med en progressiv fenotype. Refusjonsberettiget bruk: Der det er utarbeidet nasjonale handlingsprogrammer/nasjonal faglig retningslinje og/eller anbefalinger fra RHF/LIS spesialistgruppe skal rekvirering gjøres i tråd med disse. Vilkår: 216 Refusjon ytes kun etter resept fra sykehuslege eller avtalespesialist. SSc-ILD hos voksne:Refusjon følger PF-ILD kriterier.
Reseptgruppe: C
Kontaktopplysninger: medinfo.no@boehringer-ingelheim.com
Se Felleskatalogen for fullstendig preparatomtale: https://www.felleskatalogen.no/medisin/ofev-boehringer-ingelheim-593183
References:
-
OFEV® summary of product characteristics. 4th of July 2024. Page 1-7, section 4.1, 4.2, 4.3, 4.4, 4.6, 4.8, page 16, table 8, page 17-22.
-
Khor YH, Farooqi M, Hambly N, Kolb M, Ryerson CJ; Austin ILD Registry and CAREPF Investigators. Patient Characteristics and Survival for Progressive Pulmonary Fibrosis Using Different Definitions. m J Respir Crit Care Med. 2023;207(1):102-105. doi:10.1164/rccm.202205-0910LE.
-
Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive interstitial lung diseases: data from the whole INBUILD trial. Eur Respir J 2022; 59: 2004538. DOI: 10.1183/ 13993003.04538-2020.
-
Swigris JJ, et al. Responsiveness and meaningful change thresholds of the Living with Pulmonary Fibrosis (L-PF) questionnaire Dyspnoea and Cough scores in patients with progressive fibrosing interstitial lung diseases. BMJ Open Resp Res 2022;9:e001167. doi:10.1136/bmjresp-2021-001167.
-
Swigris JJ, Cutts K, Male N et al. The living with pulmonary fibrosis questionnaire in progressive fibrosing interstitial lung disease. ERJ Open Res 2021:00145-2020.
-
Varone F et al. Progressive fibrosing interstitial lung disease: a proposed integrated algorithm for management. Ann Am Thorac Soc 2020;17:1199–203.
-
Cottin, V., Richeldi, L., Rosas, I. et al. Nintedanib and immunomodulatory therapies in progressive fibrosing interstitial lung diseases. Respir Res 22, 84 (2021). doi: 10.1186/s12931-021-01668-1.
-
Raghu G et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2022;205:e18–e47.
-
Bendstrup E. et al. Nintedanib in Idiopathic Pulmonary Fibrosis: Practical Management Recommendations for Potential Adverse Events. Respiration 2019;97:173-184. doi: 10.1159/000495046
-
Richeldi L, Costabel U, Selman M, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011; 365(12): 1079-1087.
-
Beslutningsforum. Protocol. Beslutningsforum; 2023 May 23. Available from: https://nyemetoder.no/moter-i-beslutningsforum-for-nye-metoder
